How India scaled up its COVID-19 Laboratory Testing Capacity
- August 19, 2022
- ENQUIRE NOW

India has been facing pandemic situations since ages. In 1817, it first experienced Cholera pandemic followed by Flu, Smallpox, Plague and many more. In 2020, it experienced the devastating situation of COVID-19. India has always tried to upgrade its techniques and methodology according to the needed requirements. Laboratory testing methods have been one of them. India has been calibrating its testing strategy as per the changing paradigm and taking into account the scope, need and capacity to rapidly scale-up tests performed each day across the country.
Improving testing capacity necessitated taking into account a slew of issues related to capacity, data monitoring, skilled human resources and supply chain constraints. To address these issues, the government implemented many policies. The available technology was used by establishing a system to generate laboratory-linked case-based data, allowing analyses at the district, state and national levels for strategic public health interventions and breaking the transmission chain.
Here’s an overview of key steps taken by ICMR to ramp up testing capacity through different phases of the pandemic.
Collaborated with private sector to boost testing capacity
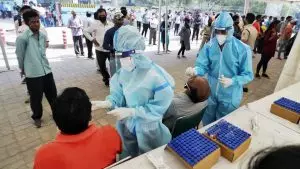
ICMR along with MoHFW onboarded certified private laboratories in India to undertake speedy laboratory testing. To ensure quality, only laboratories approved by National Accreditation Board Testing and Calibration (NABL – ISO15189) were engaged. A total of 1250 laboratories were registered with NABL for various scopes, of which labs with scope of molecular virology were chosen. Virtual laboratories tours, fast-track approvals and other measures ensured that the laboratories established followed due diligence to safeguard quality testing and reporting of samples. More than 400 private sector laboratories successfully came on board for testing within 6 months of pandemic.
Established mentor institutes for upscaling testing
To rapidly expand testing facilities, 14 Centres of Excellence were set up in different Medical Institutes, as per the directives of the Union Ministers of Home and Health & Family Welfare and under the guidance of the Director General, ICMR and Director – AIIMS (Delhi). These centres mentored government and private medical colleges in their catchment areas and eventually created a molecular virology laboratory network. In the initial phase, around 300 medical colleges across India were able to upscale COVID-19 testing over a fairly short time span.
Built capacity of scientific and technical institutions
ICMR engaged with MoHFW laboratories, Council of Scientific and Industrial Research (CSIR), Department of Biotechnology (DBT), Defence Research and Development Organisation (DRDO), Indian Council of Agricultural Research (ICAR), various government medical colleges, and high-quality private laboratories to intensify testing facilities. Remote areas were mapped proactively, so that underserved areas could be adequately covered. ICMR set up molecular diagnostic laboratories in tough terrains such as Ladakh, Arunachal Pradesh, Sikkim, Tripura, Mizoram, Lakshadweep and Andaman and Nicobar Islands to ensure that no one was left behind.
Expanded outreach through technological innovations
Indigenous point of care tests like TrueNat for COVID-19 testing that require minimal training, skills, infrastructure and biosafety measures were deployed for diagnosing COVID-19 at district facilities and primary health centres. Widespread availability of customized cartridges of TrueNat and CBNAAT serve as a big boost to take the COVID-19 testing to the level of the primary health care system. These tools have transformed testing rates, given their quick turnaround time (30-60 minutes).
It was mentioned in ‘The Launcet’ that TrueNat’s innovative methodology has been a revolutionary step regarding COVID-19 testing, especially in the underserved areas where immediate testing is required in emergency departments in India.
Safeguarded quality assurance by setting up quality control processes
ICMR identified 30 quality control (QC) laboratories to verify results of other laboratories in their respective catchment areas. Testing laboratories in each state were instructed to send positive and negative samples selected randomly to the nearest designated QC laboratories for checking and approval. The data entry portal was developed to provide uniform laboratories resulting in reporting and data management.
Strengthened regulations around 16 validation diagnostics centers

Validation centres were established for evaluation of RT-PCR kits, RNA extraction kits, VTM kits and ELISA kits. Standardized scientific protocols were followed and Indigenous manufacturers were supported by various ministries and licensure procedures were fast-tracked. To ensure transparency, follow-ups and rapid approvals, an online portal for kit validation was rolled out and more than 700 diagnostic kits have been validated since then.
India has been continuously modifying its testing strategies as required and also expand the quantity to collectively fight through the pandemic situation. Technology landscaping, leveraging resources and optimizing the processes to increase efficiency were the key approaches that the Indian Council of Medical Research (ICMR), Ministry of Health & Family Welfare (MoHFW), Government of India undertook to expand the scale of testing. The government took a quantum leap by consolidating its resources, investing in infrastructure, forging public-private partnerships, and training human resource to cater to the need for increased tests per day.