What are the hazards of HF and other glass-attacking reagents?
- September 2, 2022
- ENQUIRE NOW

What is hydrogen fluoride?
Hydrogen fluoride is a chemical compound that contains fluorine. It can exist as a colorless gas or as a fuming liquid, or it can be dissolved in water. When hydrogen fluoride is dissolved in water, it may be called hydrofluoric acid. Hydrogen fluoride can be released when other fluoride-containing compounds such as ammonium fluoride are combined with water.
How could you be exposed to hydrogen fluoride?

If you work as a Lab technician that uses hydrogen fluoride, you may be exposed to this chemical in the workplace. In case of a natural disaster, you could be exposed to high levels of hydrogen fluoride when storage facilities or containers are damaged and the chemical is released.
What are the hazards of hydrogen fluoride when in contact with human body?
- Hydrogen fluoride easily penetrates through the skin and damages the cells & tissues.
- The seriousness of poisoning caused by hydrogen fluoride depends on the amount, route, and length of time of exposure, as well as the age and pre-existing medical condition of the person exposed.
- Breathing hydrogen fluoride can damage lung tissue and cause swelling and fluid accumulation in the lungs (pulmonary edema).
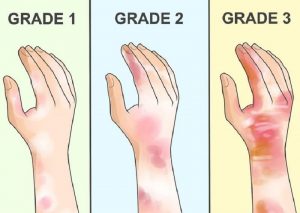
- Skin contact with hydrogen fluoride may cause severe burns that develop after several hours and form skin ulcers.
- Swallowing only a small amount of highly concentrated hydrogen fluoride will affect major internal organs and may be fatal.
- Hydrogen fluoride gas, even at low levels, can irritate the eyes, nose, and respiratory tract. Breathing in hydrogen fluoride at high levels or in combination with skin contact can cause death from an irregular heartbeat or fluid build-up in the lungs.
- Even small splashes of high-concentration hydrogen fluoride products on the skin can be fatal. Skin contact with hydrogen fluoride may not cause immediate pain or visible skin damage (signs of exposure).
- Depending on the concentration of the chemical and the length of time of exposure, skin contact with hydrogen fluoride may cause severe pain at the point of contact; a rash; and deep, slow-healing burns. Severe pain can occur even if no burns can be seen.
- Exposure to hydrogen fluoride can result in severe electrolyte problems.
Long-term health effects of acute exposure to hydrogen fluoride
- People who survive after being severely injured by breathing in hydrogen fluoride may suffer lingering chronic lung disease.
- Skin damage caused by concentrated hydrogen fluoride may take a long time to heal and may result in severe scarring.
- Fingertip injuries from hydrogen fluoride may result in persistent pain, bone loss, and injury to the nail bed.
- Eye exposure to hydrogen fluoride may cause prolonged or permanent visual defects, blindness, or total destruction of the eye.
- Swallowing hydrogen fluoride can damage the oesophagus and stomach. The damage may progress for several weeks, resulting in gradual and lingering narrowing of the oesophagus.
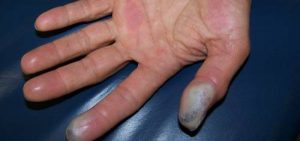
Hydrofluoric acids are considered to be very poisonous and highly corrosive in nature. Hydrofluoric Acid has many side effects and hazards to the environment as well as to human health. All the effects that have been mentioned here might not appear immediately after direct exposure to the chemical to the skin but it takes someone to show its effects and is delayed which might have the chances of being more dangerous and harmful for life.
The aqueous solution will have an average pH of 1.0.
Other Glass Attacking Reagents
Glass is always known to provide strong resistance to almost all acids. Some acids have proven to be very reactive toward the glass and are known as glass-attacking reagents. These reagents cause significant harm to the glass. These acids are Hydrofluoric acid, phosphoric acid and other phosphorous acids. The attacking reagents are those species which bring about a change in a chemical reaction.
While glass provides excellent resistance to most acids, there are three types which cause significant damage in the form of corrosion – hydrofluoric acid, phosphoric acid and phosphorus acid.
First Aid Precautions:
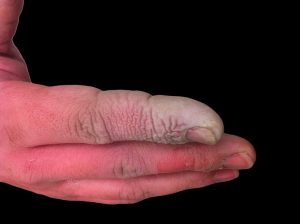
Eye: If this chemical comes in contact with the eyes, immediately wash the eyes with large amounts of water, occasionally lifting the lower and upper lids. Get medical attention immediately.
Skin: If this chemical comes in contact with the skin, dust it off immediately and then flush the contaminated skin with water. If this chemical or liquids containing this chemical penetrate the clothing, promptly remove the clothing and flush the skin with water. Get medical attention immediately.
Breath: If a person breathes large amounts of this chemical, move the exposed person to fresh air at once. If breathing has stopped, perform artificial respiration. Keep the affected person warm and at rest. Get medical attention as soon as possible.
Precautions to be taken while working with hydrofluoric acids in labs, a few steps should always be kept in mind:
- Hydrogen fluoric acid can always be neutralised by covering the small spills that are caused by it with magnesium sulphate (dry) and absorbing it with some spill control pads.
- Sodium carbonate can easily be added to the magnesium oxide in any plastic container to avoid spills and for disposal.
- To neutralise the spill, pour sodium bicarbonate solution into that area.
- Another way of avoiding the harm caused by hydrogen fluoride acid in labs is using some personal protective clothing while using it and performing experiments.
LENTUS® Bottle Top Dispensers for dispensing Hydrofluoric Acid, high-purity media and acids & bases for Trace Analysis

The Microlit LENTUS® is engineered with perfection to be used with highly corrosive acids like Hydrofluoric acid, nitric acid, sulfuric acid and many others. While conducting experiments, the LENTUS® Bottle Top Dispenser exhibits top-notch chemical compatibility and gives accurate results. This bottle top dispenser is mainly used for handling and dispensing high-purity media along with acids and bases. It is available in five unique volume ranges.
It is incorporated with Springless Valve® and Recirculation Valve technology. This advanced acid Bottle Top Dispenser is an ideal choice that prevents the loss of chemicals or reagents while performing experiments. It helps in achieving accuracy with reliability in labs.
This Hydrofluoric acid bottle-top dispenser from Microlit promotes jam-free functioning. It also comes with two product innovations, EasyKnob® and FlexiNozzle®, contributing to flexibility and ease of use.
Features of LENTUS® Bottle Top Dispenser:
While handling harsh chemicals like Hydrofluoric acids, nitric acid, sulfuric acid, and hydrochloric acid, the LENTUS® bottle top dispenser can be used to facilitate a wide range of actions.
Change Volume with EasyKnob®
It allows easy 180° rotation for effortless volume setting.
Move effortlessly with a PTFE Piston.
It features a PTFE Piston along with the ETP O-Ring and ensures effortless and smooth movement. It also makes sure of high chemical compatibility.
Re-direct Liquids with Recirculation Valve
It prevents any loss of reagents when purging takes place by re-directing it into the mounted bottle. It also facilitates no-bubble dispensing.
Operate Jam-free with Springless Valve®
It features an award-winning technology called Springless Valve® which ensures easy and jam-less movements.

Dispense Easily with FlexiNozzle®
It comes with another promising technology, FlexiNozzle®, an adjustable nozzle that ensures easy flexibility and facilitates easy and smooth dispensing.
Work Flexibly with a 360° Adapter.
It allows 360° rotation ensuring complete flexibility and easy movements.
Assured Quality with ISO 8655 Conformed Calibration
According to ISO 8655 standards, it is calibrated in an ISO 17025 accredited laboratory.
Work with a Comprehensive Volume Range
It is primarily available in five broad volume ranges of 0.25-2.5 ml, 0.5-5 ml, 1-10 ml, 2.5-30 ml, 5-60 ml, 10-100 ml.
Autoclaving Parameters
It is autoclavable at 15 psi and 121°C for 15-20 minutes.
Designed with utmost care and high ergonomics, this bottle top dispenser offers excellent chemical compatibility, especially with hydrofluoric acid or HF. Not just hydrofluoric acid, it works well with other chemicals and reagents to achieve precision while taking care of safety in real laboratory environments. Besides ensuring safety in the laboratory, this bottle-top dispenser can be used to facilitate a broad range of applications and boost reliability. While working in labs, professionals take appropriate measures to conduct experiments, ensuring safety, especially while working with hazardous chemicals. To know more about this unique product, visit our website https://www.microlit.com/product/microlit-lentus/